Jul 8 2015
About 42 miles southwest of San Francisco and 2,600 feet underwater sits the U.S.S. Independence, a bombed-out relic from World War II. The aircraft carrier was a target ship in atomic weapon tests at Bikini Atoll in the Marshall Islands during the war. Then, in 1951, it was loaded up with 55-gallon drums of low-level radioactive waste and scuttled just south of the Farallon National Wildlife Refuge off the California coast.
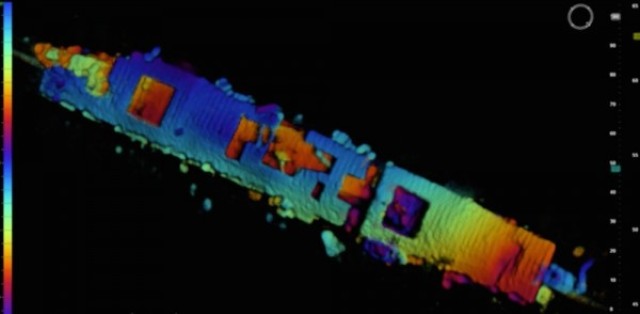 An image of the USS Independence from the Coda Octopus Echoscope 3D sonar, which was integrated on the Boeing Autonomous Underwater Vehicle (AUV) Echo Ranger. Credit: NOAA and Coda Octopus
An image of the USS Independence from the Coda Octopus Echoscope 3D sonar, which was integrated on the Boeing Autonomous Underwater Vehicle (AUV) Echo Ranger. Credit: NOAA and Coda Octopus
Earlier this year, the U.S.S. Independence was rediscovered by a team of researchers led by James Delgado, the director of Maritime Heritage at the National Oceanic and Atmospheric Administration (NOAA). The marine archaeologists used sonar from an autonomous submarine to find the wreckage, but with the ship’s radioactive past, the scientists wondered how safe it would be to actually explore.
Delgado turned to Berkeley Lab’s Kai Vetter to better understand the radiation hazards. Vetter is the head of applied nuclear physics at Berkeley Lab, nuclear engineering professor at the University of California, Berkeley, and the co-founder of the Institute for Resilient Communities. “They wanted to know if we could ensure the safety of their equipment,” says Vetter, “and to see if you’d pick up contamination if you went down there.”
The short answers, Vetter says, was that neither the submersible nor the team was ever in danger of contamination.
One reason is that water is an excellent radiation shield. Under water, radiation will only extend several inches from contaminated materials, says Vetter. The unmanned research submarine stayed at least 100 feet away from the wreck.
Another reason has to do with the size of the contaminated site with respect to the size of the ocean. While contaminated rust particles from the ship are released and transported by water, the dilution factor of the ocean is enormous, essentially nullifying any radioactive effect.
Relatedly, while a relatively small number of organisms close to the wreck might take up some of these rust particles, the effects of radioactivity are diluted through the food chain because the number of organisms exposed is so small. In contrast, mercury is much more prevalent and widely distributed in the ocean, and this is why its concentration builds up the food chain.
And finally, says Vetter, it’s important to consider the half-life of the radioactive materials. In this case, the isotopes of concern are cesium 137 and strontium 90, which both have a half-life of about 30 years. This means that after 30 years, half the isotopes responsible for the initial contamination transmute into other non-radioactive isotopes. It’s been over 60 years since the U.S.S Independence was scuttled, which means that less than a quarter of the initial radioactive isotopes remain.
Still, to demonstrate with data, Vetter brought a team of researchers and students to the harbor in Half Moon Bay, CA to test the submersible after it had captured sonar images of the aircraft carrier. Armed with instruments called dosimeters that pick up ionizing radiation, the researchers found no evidence of contamination on the submersible. It wasn’t a surprise, says Vetter, since the craft never got close enough to the ship and even if it had, the contamination would have diluted away as it was tugged back to shore.
The NOAA expedition collected its sonar images from a distance, but Vetter hopes to someday work with a submersible that gets an up-close view of the ship, the 55-gallon barrels, and the radioactivity. Such a project would require a specially designed detector to read the radiation on site, Vetter explains. “It would be exciting to build a dedicated system with some advanced technologies to figure out what is sitting down there in that old vessel,” he says.
For more information about the U.S.S. Independence go here.
For more information about Kai Vetter go here.
For more information about the Institute for Resilient Communities go here.