Sep 24 2014
Pietro Valdastri, assistant professor of mechanical engineering at Vanderbilt University, and colleagues will continue to develop a unique endoscope for colonoscopy in patients with inflammatory bowel disease with the support of a 4-year, $1.5 million grant – “A magnetic capsule endoscope for colonoscopy in patients with IBD” – from the National Institute of Biomedical Imaging and Bioengineering, one of the 26 National Institutes of Health.
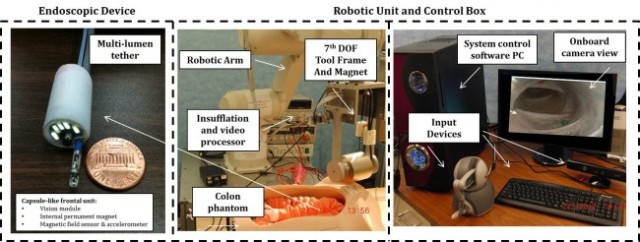
“Capsule endoscopy has already been extremely helpful for IBD patients, especially in the diagnosis of Crohn’s disease in children and young adults. Adapting capsule endoscopy to colonoscopy – as proposed by this project – would allow for reduced risk of adverse events and provide a great benefit to patients with IBD,” said Valdastri, the grant’s principal investigator and director of the School of Engineering’s Science and Technology of Robotics in Medicine (STORM) laboratory. Keith L. Obstein, assistant professor, Division of Gastroenterology, Hepatology and Nutrition at the Vanderbilt University Medical Center, is co-PI.
Using the robotic platform proposed by the researchers, an endoscopist will be able to remotely control the motion of a soft-tethered capsule to perform diagnosis and treatment at any location inside the human colon.
Manipulation of the capsule is achieved by magnetic coupling, thanks to the interaction of two permanent magnets – one integrated inside the capsule and the other placed at the end effector of a robotic arm. Navigating the endoscope using a magnet allows for pulling the capsule endoscope from the front rather than pushing the traditional colonoscope tube from the back, which can lead to painful stretching of the colon.
“Leveraging extensive preliminary results, with this study we will develop a fully vetted system ready for human clinical trials, and we will test the hypothesis that our approach has diagnostic and therapeutic capabilities comparable to standard colonoscopy,” Valdastri said.
If successful, Valdastri says this approach will widen the implementation of IBD surveillance programs to rural areas thanks to the low cost of the platform – less than $20,000 – and the ease of transport. There will be no need for anesthesiologists or sedation monitors, and no need for specialized facilities where examinations are performed.
The investigative team includes engineering and clinical faculty at Vanderbilt. Valdastri said the team “collectively possesses expertise in endoscopic device design, clinical colonoscopy, assessment and validation of innovative, and are uniquely positioned to achieve success.”