Jun 13 2016
A team of researchers from Osaka University and Tohoku University has created a new robot microscope system that is capable of automatically tracking a freely moving small animal and controlling its brain activity through "projection mapping."
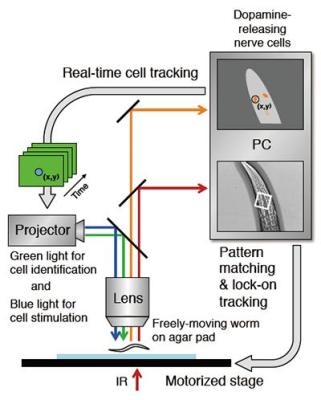 Schematic drawing of the robot microscope system for optical manipulation of a nerve cell. (Adapted from Tanimoto et al., Scientific Reports) (Photo Credit: Scientific Reports)
Schematic drawing of the robot microscope system for optical manipulation of a nerve cell. (Adapted from Tanimoto et al., Scientific Reports) (Photo Credit: Scientific Reports)
The researchers have named the system as OSaCaBeN (OSB). They analyzed the nematode C. elegans (a roundworm) using the robot-scope. The roundworm is often used for studying basic brain functions. The team showed the nerve cells’ functional diversification during release of dopamine. Dopamine is a chemical that controls emotion, movement, and motivation in the animal brain.
In order to comprehend the workings of the brain, the activities of nerve cells found in the brain have to be measured, and a hypothesis is drawn based on the manner in which the activity of a specific nerve cell is linked to the operation of the brain. The hypothesis is then analyzed by artificially controlling the activity of the nerve cell and monitoring its effect on the behavior of the animal, the most important output of brain function.
Due to the latest advances in genetic engineering methods, activities of particular nerve cells can be optically measured and controlled under a microscope. Understanding the connection between the activity of a specific nerve cell and the behavior of the animal continues to be a challenge due to the intricacy of an animal's brain.
Neuroscientists frequently use the C. elegans as it has a tiny brain made up of only 302 nerve cells. Despite that, it still can respond to a variety of stimuli by applying molecules that are very similar to the ones in other animals, including humans. Furthermore, the transparent body of the C. elegans enables the nerve cell activities to be easily measured and controlled using the above-mentioned optical methods.
However, it is quite a task to test the neural activities of moving worms. The worms move ~0.1 mm per second, which is highly cumbersome to pursue as they cross over the field of vision of a microscope in just one second.
The research team led by Prof. Koichi Hashimoto solved the issue by creating a robot-scope that has the ability to automatically track a worm on a stage with advanced software technology known as "machine-vision."
The robot-scope is designed to locate a part of the worm's head from the total image, and alters the position of the microscope stage to constantly maintain the head at the center of the field of vision with an accuracy of ±0.001 mm.
Although such a process of image identification usually takes several hours, the robot scope does it 200 times per second. This allows us to optically measure the continuous activities of multiple nerve cells in a worm's brain as it is moving.
Professor Koichi Hashimoto, Tohoku University
Also, in a somewhat different arrangement, the system can locate and track a single specific nerve cell from a bunch of cells in a moving worm using another machine-vision software. It can manipulate its activity with the nonstop illumination of a fine light beam. This robot microscope is the first of its kind to be able to perform manipulation of nerve cells and optical measurement with such a good level of accuracy.
Prof. Kotaro Kimura's team was able to show functional diversification of dopamine-releasing nerve cells using the robot-scope. In worms, dopamine is released from four pairs of nerve cells when they reach food (a lawn of bacteria). Dopamine also alters signal sensations, movement of numerous parts of their body, and learning.
It is yet to be discovered how these food signal, which is the most crucial information for worms to survive, translate into activities of the dopamine-releasing nerve cells. The researchers showed that just the dorsal pair of dopamine-releasing neurons (CEPD) is considerably activated when food is reached. Additionally, artificial activation of CEPD resulted in behavioral modifications matching those observed when the food is reached.
However, it was noticed that structurally similar dorsal pair of dopamine-releasing nerve cells (CEPV) was not activated when it reached the food. Artificial activation of CEPV did not result in the behavioral alterations similar to the activation of CEPD. It is likely that CEPV is activated in a diverse situation and plays a varied role in behavioral modulation.
Therefore, the researchers discovered that even structurally symmetric dopamine-releasing neurons possessed asymmetric operations.
We will analyze more of the relationships between brain and behavior using the robot microscope system on worms as well as zebrafish. We would like to understand the basic principles of brain function through the analyses of these simple animals.
Professor Kotaro Kimura, Osaka University