Apr 6 2016
Ekso Bionics Holdings, Inc., a robotic exoskeleton company, today announced that it has received clearance from the U.S. Food and Drug Administration (FDA) to market its Ekso GT robotic exoskeleton for use in the treatment of individuals with hemiplegia due to stroke, individuals with spinal cord injuries at levels T4 to L5, and individuals with spinal cord injuries at levels of T3 to C7 (ASIA D), in accordance with device’s labeling. The Ekso GT is the first exoskeleton cleared by the FDA for use with stroke patients.
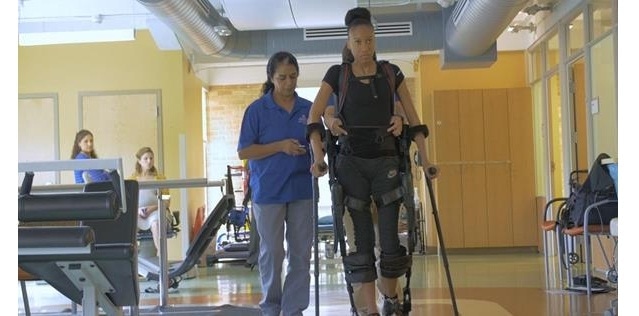 Ekso GT™ Robotic Exoskeleton
Ekso GT™ Robotic Exoskeleton
Ekso GT is a wearable robotic exoskeleton that enables individuals to stand up and walk over ground with a full weight bearing, reciprocal gait in a clinical setting. The Ekso GT with smart Variable Assist* software, which was designed for rehabilitation institutions, provides adaptive amounts of power to either side of the patient’s body, engaging the patient throughout his or her continuum of care. The technology provides the ability to mobilize patients early in their recovery, frequently, with a significant number of high intensity steps. To date, the Ekso has helped patients take more than 41 million steps in over 115 rehabilitation institutions around the world.
“This clearance marks a major milestone towards our goal of establishing exoskeletons as standard of care in the rehabilitation clinic," commented Thomas Looby, president and interim chief executive officer of Ekso Bionics. “Our strategy has been to concentrate on the rehabilitation clinic, with a focus on ease of use, rapid turn over between sessions, and efficacy for a range of patients. Clinics using the Ekso GT are able to offer exoskeleton therapy to the widest patient population among all exoskeletons on the market, which we believe will translate into broader adoption of exoskeletons by hospitals and rehabilitation clinics and better outcomes for patients.”
Each year, an estimated 375,000 people suffer a spinal cord injury globally and an estimated 17 million people suffer a stroke. Over 60% of acute stroke survivors are unable to walk or need intervention in walking. Impaired ambulation is greatly associated with fall risks, dependency, limited participation in social activities, and poor quality of life. As a consequence, assisting with ambulation in the clinical environment may aid in the recovery of ambulation that is one of the most desired goals for stroke survivors undergoing rehabilitation.
"We appreciate the collaboration with the leading rehabilitation institutions who helped contribute to our submission,” added Mr Looby.
"I congratulate Ekso Bionics for being the first exoskeleton to receive clearance for stroke,” said W. Zev Rymer, Director, Research Planning and Sensory Motor Performance Program, Rehabilitation Institute of Chicago. "When we partnered with Ekso at the beginning of 2012, they had the first exoskeleton that was uniquely optimized for the rehabilitation clinic. We have seen the clinical value of the technology, and Ekso Bionic's continued innovation now brings us the ability to provide this advanced technology to a broader patient population."