Oct 5 2015
A JRC invention initially stemming from its research in the nuclear sector will soon be used by hospitals for minimally-invasive robotic surgery. TELELAP ALF-X is an advanced multi-port robotic system that will empower surgeons with new technologies such as eye-tracking and haptics, allowing them eye-control of the camera and touch sensation during surgery. Hospitals will be able to use the most advanced technology while running at low operational costs, as the system can use current surgical instruments.
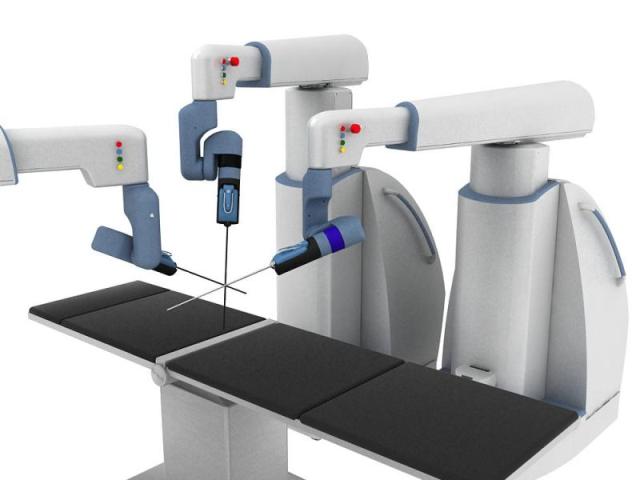 The main features are tactile sensing, versatile & user-friendly robotic arms, comfortable & ergonomic surgeon console, and eye-tracking. © EU, 2015
The main features are tactile sensing, versatile & user-friendly robotic arms, comfortable & ergonomic surgeon console, and eye-tracking. © EU, 2015
ALF-X, which stands for "Advanced Laparoscopy through Force refleCTion", was initially developed in 2003-2009 by Emilio Ruiz Morales, a Spanish engineer who at the time worked on nuclear safeguards at the JRC. The system employs a range of features for easier, safer and improved laparoscopic surgical procedures for the benefit of the patient. The main features are tactile sensing, versatile & user-friendly robotic arms, comfortable & ergonomic surgeon console, and eye-tracking. For instance, tactile sensing provides surgeons with better perception of forces exerted on patient tissues by robotic surgical instruments, enabling them to perform accurate and delicate tissue manipulations. The system also limits applied forces, avoiding breaking sutures (surgical stitching) and reducing any damage caused to the incision by surgical tools used in keyhole operations. Moreover, the robotic arms are easily operated and quickly configured by surgical theatre personnel.
This new technology is built on the GENERIS software that was used for controlling mechanical "arms" to manipulate highly radioactive material in storage areas. The JRC-owned patents were initially licensed to the Italian company Sofar in 2008. Sofar invested in more research and development, CE-Mark certification and production set-up, with the technical direction of Emilio Ruiz Morales . To further fund product research and to market this medical device on a worldwide-scale, Sofar now works with TransEnterix Inc, a worldwide leader in robotic surgery. A new company has been set up for this purpose and will soon be renamed "TransEnterix Italia". The device obtained the CE Mark in 2012.
The new licence agreement, renegotiated by the JRC, will bring royalties over the course of its duration (i.e. until 2027), which will be reinvested in JRC's research.