Aug 12 2013
Surgery to relieve the damaging pressure caused by hemorrhaging in the brain is a perfect job for a robot.
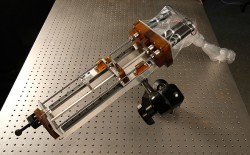 Steerable needle robot (Joe Howell / Vanderbilt)
Steerable needle robot (Joe Howell / Vanderbilt)
That is the basic premise of a new image-guided surgical system under development at Vanderbilt University. It employs steerable needles about the size of those used for biopsies to penetrate the brain with minimal damage and suction away the blood clot that has formed.
The system is described in an article accepted for publication in the journal IEEE Transactions on Biomedical Engineering. It is the product of an ongoing collaboration between a team of engineers and physicians headed by Assistant Professor Robert J. Webster III and Assistant Professor of Neurological Surgery Kyle Weaver.
Robot treats brain clots with steerable needles
Brain clots are leading cause of death, disability
The odds of a person getting an intracerebral hemorrhage are one in 50 over his or her lifetime. When it does occur, 40 percent of the individuals die within a month. Many of the survivors have serious brain damage.
“When I was in college, my dad had a brain hemorrhage,” said Webster. “Fortunately, he was one of the lucky few who survived and recovered fully. I’m glad I didn’t know how high his odds of death or severe brain damage were at the time, or else I would have been even more scared than I already was.”
Steerable needle could prevent “collateral damage” during surgery
Operations to “debulk” intracerebral hemorrhages are not popular among neurosurgeons: They know their efforts are not likely to make a difference, except when the clots are small and lie on the brain’s surface where they are easy to reach. Surgeons generally agree that there is a clinical benefit from removing 25-50 percent of a clot but that benefit can be offset by the damage that is done to the surrounding tissue when the clot is removed. Therefore, when a serious clot is detected in the brain, doctors take a “watchful waiting” approach – administering drugs that decrease the swelling around the clot in hopes that this will be enough to make the patient improve without surgery.
For the last four years, Webster’s team has been developing a steerable needle system for “transnasal” surgery: operations to remove tumors in the pituitary gland and at the skull base that traditionally involve cutting large openings in a patient’s skull and/or face. Studies have shown that using an endoscope to go through the nasal cavity is less traumatic, but the procedure is so difficult that only a handful of surgeons have mastered it.
Last summer, Webster attended a conference in Italy where one of the speakers, Marc Simard, a neurosurgeon at the University of Maryland School of Medicine, ran through his wish list of useful imaginary neurosurgical devices, hoping that some engineer in the audience might one day be able to build one of them. When he described his wish to have a needle-sized robot arm to reach deep into the brain to remove clots, Webster couldn’t help smiling because the steerable needle system he had been developing was perfect for the job.
Webster’s design, which he calls an active cannula, consists of a series of thin, nested tubes. Each tube has a different intrinsic curvature. By precisely rotating, extending and retracting these tubes, an operator can steer the tip in different directions, allowing it to follow a curving path through the body. The single needle system required for removing brain clots was actually much simpler than the multi-needle transnasal system.
When Webster returned, he told Weaver about the potential new application. The neurosurgeon was quite supportive: “I think this can save a lot of lives. There are a tremendous number of intracerebral hemorrhages and the number is certain to increase as the population ages.”
Graduate student Philip Swaney, who is working on the system, likes the fact it is closest to commercialization of all the projects in Webster’s Medical and Electromechanical Design Laboratory. “I like the idea of working on something that will begin saving lives in the very near future,” he said.
Active cannula removed 92 percent of clots in simulations
The brain-clot system only needs two tubes: a straight outer tube and a curved inner tube. Both are less than one twentieth of an inch in diameter. When a CT scan has determined the location of the blood clot, the surgeon determines the best point on the skull and the proper insertion angle for the probe. The angle is dialed into a fixture, called a trajectory stem, which is attached to the skull immediately above a small hole that has been drilled to enable the needle to pass into the patient’s brain.
The surgeon positions the robot so it can insert the straight outer tube through the trajectory stem and into the brain. He also selects the small inner tube with the curvature that best matches the size and shape of the clot, attaches a suction pump to its external end and places it in the outer tube.
Guided by the CT scan, the robot inserts the outer tube into the brain until it reaches the outer surface of the clot. Then it extends the curved, inner tube into the clot’s interior. The pump is turned on and the tube begins acting like a tiny vacuum cleaner, sucking out the material. The robot moves the tip around the interior of the clot, controlling its motion by rotating, extending and retracting the tubes. According to the feasibility studies the researchers have performed, the robot can remove up to 92 percent of simulated blood clots.
“The trickiest part of the operation comes after you have removed a substantial amount of the clot. External pressure can cause the edges of the clot to partially collapse making it difficult to keep track of the clot’s boundaries,” said Webster.
The goal of a future project is to add ultrasound imaging combined with a computer model of how brain tissue deforms to ensure that all of the desired clot material can be removed safely and effectively.
Other members of the research team are Jessica Burgner, formerly a postdoctoral fellow at Vanderbilt and now executive director of the Hannover University Center for Mechatronics in Germany, and Ray Lathrop, a graduate student at Vanderbilt.
The research was supported by National Science Foundation CAREER Award IIS-1054331 and Graduate Research Fellowship as well as a grant from the German Academic Exchange Service.