Mazor Robotics Ltd has received approval from the US Food and Drug Adminstration (FDA) and the the EU CE Mark certification for its new variation of the SpineAssist robot. The next generation robot for spinal surgery is the Israeli company’s addition to its Renaissance product.
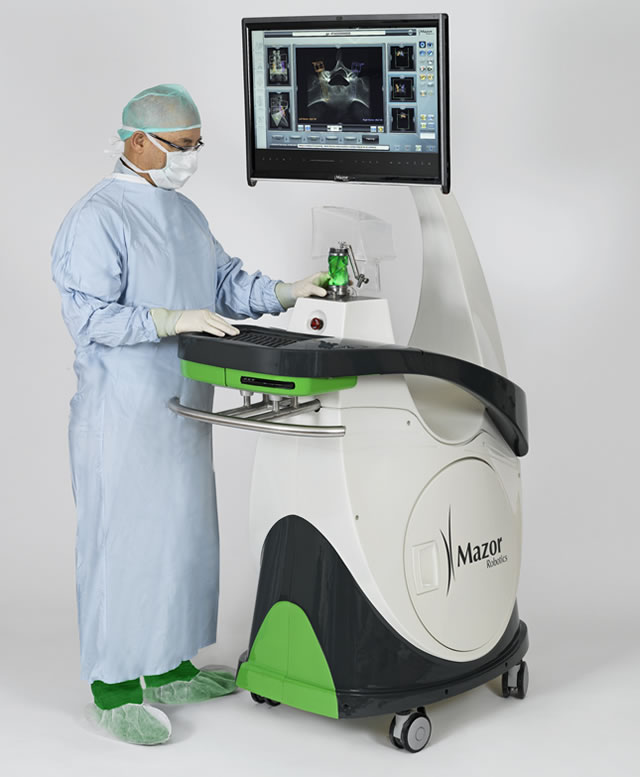 Mazor Robotics’ Renaissance is transforming spine surgery. Image Credit: Mazor Robotics
Mazor Robotics’ Renaissance is transforming spine surgery. Image Credit: Mazor Robotics
The Renaissance robot has the capability to assist surgeons with a wide range of operating procedures which include complex spine surgeries and robotic guided cranial surgeries. The company said that sales for the robot will begin immediately now that the approvals have been granted.
The old SpineAssist robot has been already used in over 2000 spinal surgeries globally and has been used in placing more than 12,000 implants. The Renaissance which has a new design and human interface hopes to take the story further with new hardware and software capabilities for the surgical robot.
The system also reduces radiation protocols for preoperative CTs by up to 50% leading to far less radiation exposure for the patients. The increased surgical safety will extend to a number of clinical applications such as enabling osteotomies, transfacet and translaminar-facet implant placements, in addition to procedures such as spinal fusions and scoliosis corrections currently performed with Mazor Robotics’ technology.
Spinal procedures that the robot can carry out will include Open, MIS, and percutaneous posterior thoracolumbar approaches; Scoliosis and other complex spinal deformities;Pedicle screws – short and long fusions; Transfacet screws and translaminar-facet screws; Osteotomies and Biopsies.