An artificial intelligence (AI)-based method for finding high-affinity antibody drugs has been formulated by a team of researchers at the University of California San Diego School of Medicine.
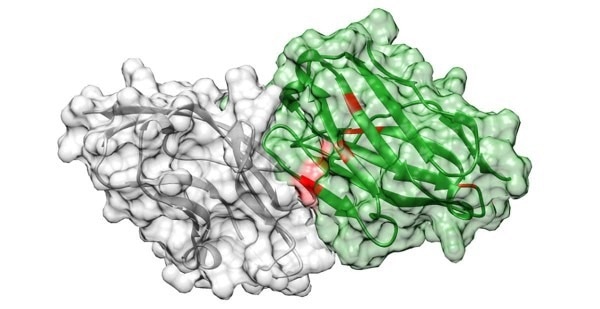
The AI-based approach successfully identified a new antibody, shown here tightly bound to its target, PD-L1. Image Credit: UC San Diego Health Sciences.
Scientists used the method to discover a new antibody that binds a key cancer target 17-fold tighter than current antibody drugs. They say the pipeline could speed up the discovery of new drugs to combat cancer and other diseases like rheumatoid arthritis and COVID-19.
Details of the study have been published in the January 28th, 2023 issue of Nature Communications.
For a drug to be successful, an antibody has to bind firmly to its target. To discover such antibodies, scientists usually begin with a well-established antibody amino acid sequence and employ bacterial or yeast cells to create a series of new antibodies with differences in that sequence.
These mutants are then assessed for their capability to bind the target antigen. The subset of antibodies that function best are then exposed to another cycle of mutations and assessments, and this cycle repeats until a set of closely-bound finalists is formed.
Regardless of this long and costly process, many of the subsequent antibodies are still not good enough to be successful in clinical trials. In the new research, UC San Diego researchers engineered an advanced machine learning algorithm to quicken and simplify these efforts.
The method begins similarly, with scientists producing a preliminary library of approximately half a million possible antibody sequences and screening them for their affinity to a particular protein target.
But rather than carrying out this process again and again, they decided to feed the dataset into a Bayesian neural network which can examine the data and use it to estimate the binding affinity of other sequences.
With our machine learning tools, these subsequent rounds of sequence mutation and selection can be carried out quickly and efficiently on a computer rather than in the lab.
Wei Wang Ph.D., Study Senior Author and Professor, Cellular and Molecular Medicine, School of Medicine, University of California-San Diego
One specific benefit of their AI model is its ability to reveal the certainty of every prediction.
Unlike a lot of AI methods, our model can actually tell us how confident it is in each of its predictions, which helps us rank the antibodies and decide which ones to prioritize in drug development.
Wei Wang Ph.D., Study Senior Author and Professor, Cellular and Molecular Medicine, School of Medicine, University of California-San Diego
To corroborate the pipeline, project researchers and co-first authors of the study Jonathan Parkinson, Ph.D., and Ryan Hard, Ph.D., aimed to engineer an antibody against programmed death ligand 1 (PD-L1), a protein extremely expressed in cancer and the target of numerous anti-cancer drugs that are currently available.
Using this method, they discovered a new antibody that was capable of binding to PD-L1 17 times better than atezolizumab (brand name Tecentriq), the wild-type antibody permitted for clinical application by the U.S. Food and Drug Administration.
Scientists are currently using this method to detect favorable antibodies against other antigens like SARS-CoV-2. They are also creating more AI models that can examine amino acid sequences for other antibody features crucial for the success of clinical trials, like solubility, stability, and selectivity.
“By combining these AI tools, scientists may be able to perform an increasing share of their antibody discovery efforts on a computer instead of at the bench, potentially leading to a faster and less failure-prone discovery process,” said Wang. “There are so many applications to this pipeline, and these findings are really just the beginning,” Wang added.
This study received funding, in part, from the National Institutes of Health (Grant Nos: R01GM111941 and R21AI158114).
Journal Reference
Parkinson, J., et al. (2023) The RESP AI model accelerates the identification of tight-binding antibodies. Nature Communications. doi.org/10.1038/s41467-023-36028-8.