Sep 10 2020
The outcomes of a multi-center, single-arm trial of the ReWalk ReStore™ for gait training in patients undergoing post-stroke rehabilitation were published by a group of U.S. researchers.
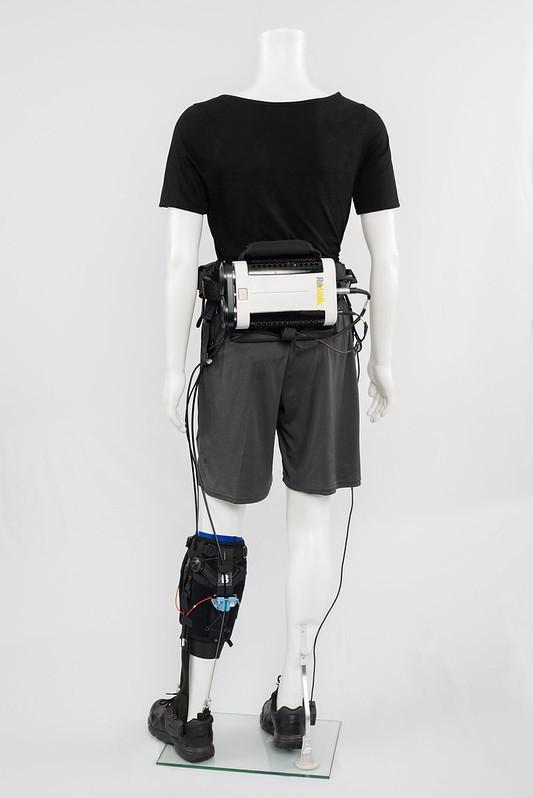 Researchers completed a multi-center, single-arm trial study of the ReStore for gait training of individuals undergoing post-stroke rehabilitation. Image Credit: ReWalk Robotics Ltd.
Researchers completed a multi-center, single-arm trial study of the ReStore for gait training of individuals undergoing post-stroke rehabilitation. Image Credit: ReWalk Robotics Ltd.
The device was found to be safe and reliable while performing treadmill and overground walking under the supervision of physical therapists.
The article titled “The ReWalk ReStore soft robotic exosuit: a multisite clinical trial of the safety, reliability, and feasibility of exosuit-augmented post-stroke gait rehabilitation” was published open access in the Journal of NeuroEngineering and Rehabilitation on June 18th, 2020.
The study authors are the principal investigators of each of the five testing sites: Louis N. Awad, PT, DPT, PhD, of Spaulding Rehabilitation Hospital, Boston, MA; Alberto Esquenazi, MD, of MossRehab Stroke and Neurological Disease Center, Elkins Park, PA; Gerard E. Francisco, MD, of TIRR Memorial Hermann, Houston, TX; Karen J. Nolan, PhD, of Kessler Foundation, West Orange, NJ; and lead investigator Arun Jayaramam, PT, PhD, of the Shirley Ryan AbilityLab, Chicago, IL.
The ReStore™ exosuit available from ReWalk Robotics, Ltd is the first-ever soft robotic exosuit approved by the FDA for stroke survivors who face mobility deficits. The device is recommended for patients with hemiplegia who undergo stroke rehabilitation under the care of licensed physical therapists.
Hemiplegia leads to weakness of the ankle, which restricts the ability to clear the ground during stepping and hampers forward movement. This results in compensatory walking patterns that reduce the stability and increase the effort.
ReStore has been engineered to reinforce ankle plantarflexion and dorsiflexion, enabling a highly normal gait pattern. Motors fitted on a waist belt transmit power via cables to attachment points on an insole and the calf of the patient. Sensors attached to the shoes of the patient transmit data to a handheld smartphone controller, which is used by a trained therapist to control levels of assistance, as well as monitor and record key gait training metrics.
As part of the trial, 44 participants with post-stroke hemiparesis enrolled and they could walk without any assistance for 5 feet. The protocol included 5 days of 20-minute treadmill and overground training sessions under the supervision of licensed physical therapists.
The researchers evaluated the therapeutic potential for ReStore in rehabilitation by exploring the effects of the device on maximum walking speed, measuring the walking speed of participants in and out of the device with the 10-m walk test, before and after the five training visits. To ensure safety, certain participants were permitted to use a cane or AFO during the walking sessions.
In the trial, the reliability, safety, and feasibility of the device in this stroke population were determined.
We found that the ReStore provided targeted assistance for plantarflexion and dorsiflexion of the paretic ankle, improving the gait pattern. This is an important first step toward expanding options for rehabilitative care for the millions of individuals with mobility impairments caused by ischemic and hemorrhagic stroke.
Dr Karen J. Nolan, Senior Research Scientist, Center for Mobility and Rehabilitation Engineering Research, Kessler Foundation
The exploratory data obtained from the trial pointed toward the positive effects of the training on the walking speed of participants at the time of unassisted walking (walking without the device) and exosuit-assisted walking. Over one-third of the participants realized a considerable increase in unassisted walking speed, which indicates that further studies are warranted.
Dr Nolan highlighted that the trial was not designed to quantify the efficacy of the device.
Controlled trials are needed to determine the efficacy of ReStore for improving mobility outcomes of stroke rehabilitation.
Dr Karen J. Nolan, Senior Research Scientist, Center for Mobility and Rehabilitation Engineering Research, Kessler Foundation
Journal Reference:
Awad, L. N., et al. (2020) The ReWalk ReStore™ soft robotic exosuit: a multi-site clinical trial of the safety, reliability, and feasibility of exosuit-augmented post-stroke gait rehabilitation. Journal of NeuroEngineering and Rehabilitation. doi.org/10.1186/s12984-020-00702-5.