May 14 2020
At the University of Toronto, Faculty of Engineering and Carnegie Mellon University, scientists are making use of artificial intelligence (AI) to speed up the progress in converting waste carbon into a commercially useful product with efficiency like never before.
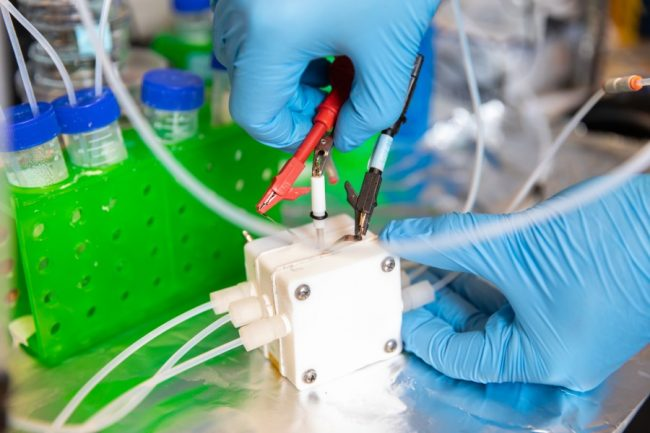 Researchers from U of T Engineering and Carnegie Mellon University are using electrolyzers like this one to convert waste CO2 into commercially valuable chemicals. Their latest catalyst, designed in part through the use of AI, is the most efficient in its class. Image Credit: Daria Perevezentsev.
Researchers from U of T Engineering and Carnegie Mellon University are using electrolyzers like this one to convert waste CO2 into commercially valuable chemicals. Their latest catalyst, designed in part through the use of AI, is the most efficient in its class. Image Credit: Daria Perevezentsev.
They utilized AI to accelerate the search for the key material in a new catalyst that transforms carbon dioxide (CO2) into ethylene—a chemical precursor to an extensive range of products, spanning from dish detergent to plastics.
The ensuing electrocatalyst is the most effective in its class. When operated with solar or wind power, the system also offers an efficient method to store electricity from renewable but intermittent sources.
Using clean electricity to convert CO2 into ethylene, which has a $60 billion global market, can improve the economics of both carbon capture and clean energy storage.
Ted Sargent, Study Senior Author and ECE Professor, Faculty of Applied Science & Engineering, University of Toronto
The study was published recently in the journal Nature.
Sargent and his colleagues have already designed several world-leading catalysts to decrease the energy charges of the reaction that transforms CO2 into ethylene and other carbon-based molecules.
However, even improved catalysts may exist, and since there are millions of prospective material combinations to choose from, testing all of them would be excessively time-consuming.
The researchers demonstrated that machine learning can speed up the search. By using theoretical data and computer models, algorithms can throw out the worst options and show more potential candidates.
The use of AI to look for clean energy materials was proposed at a 2017 workshop that was organized by Sargent in collaboration with the Canadian Institute for Advanced Research (CIFAR). The concept was further developed in a Nature commentary article that was published later that year.
Professor Zachary Ulissi from Carnegie Mellon University was one of the scientists invited at the original workshop. His team specializes in computer modeling of nanomaterials.
With other chemical reactions, we have large and well-established datasets listing the potential catalyst materials and their properties. With CO2-to-ethylene conversion, we don’t have that, so we can’t use brute force to model everything. Our group has spent a lot of time thinking about creative ways to find the most interesting materials.
Zachary Ulissi, Professor, Carnegie Mellon University
The algorithms that were developed by Ulissi and his colleagues make use of a combination of machine learning models and active learning methods to widely estimate the types of products a given catalyst can produce, even without in-depth modeling of the material itself.
The researchers applied the algorithms for the reduction of CO2 to analyze 240 different materials, thus identifying four potential candidates that were estimated to have useful properties over an extensive range of surface structures and compositions.
In the recent study, the co-authors characterize their best-performing catalyst material, which is an alloy of aluminum and copper. Following the bonding of the two metals at a high temperature, a small portion of the aluminum was then etched away, leading to a nanoscale porous structure that Sargent defines as “fluffy.”
Furthermore, the new catalyst was tested in a device known as an electrolyzer, and the “faradaic efficiency”—the proportion of electrical current used to make the preferred product—was quantified at 80%, which is a new record for this reaction.
According to Sargent, the cost of energy should be reduced even more if the system has to generate ethylene that is cost-competitive with that extracted from fossil fuels. The focus of further studies will be to decrease the overall voltage needed for the reaction, as well as bringing down the proportion of side products further, which are expensive to isolate.
The new catalyst is the first-ever one designed for CO2-to-ethylene transformation partially using AI. Also, it is the initial experimental illustration of the active learning methods that have been developed by Ulissi. Its powerful performance verifies the effectiveness of this approach and is a good sign for future collaborations of this kind.
There are many ways that copper and aluminum can arrange themselves, but what the computations shows is that almost all of them were predicted to be beneficial in some way,” says Sargent. “So instead of trying different materials when our first experiments didn’t work out, we persisted, because we knew there was something worth investing in.
Ted Sargent, Study Senior Author and ECE Professor, Faculty of Applied Science & Engineering, University of Toronto
Journal Reference:
Zhong, M., et al. (2020) Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature. doi.org/10.1038/s41586-020-2242-8.